Continue from previous page
Light-driven rotary nanomotors
The first artificial rotary nanomotor was developed in 1999 by Ben Feringa’s group at the University of Groningen in the Netherlands (Koumura 1999)1Koumura, N., Zijlstra, R. W. J., van Delden, R. A., Harada, N., Feringa, B. L. (1999) Light-driven monodirectional molecular rotor, Nature, 401: 152-155.. The motor, illustrated in Fig. 26, is operated only by light. In the same way as compounds having a –N=N– group (Fig. 18), also those containing a –C=C– group (for example, stilbene, Fig. 26a) may exist as trans and cis isomers. In systems of this type, light excitation of one of the two isomers – for example, the trans one – can cause the 180° rotation of one of the two molecular units with respect to the other, with formation of the cis isomer. The excitation of the latter can then cause the return to the initial trans isomer, through a sub-sequent 180° rotation. In simple compounds such as stilbene, the direction of the rotary motion is random, so it cannot be taken for granted that the trans → cis → trans transformation occurs through a complete rotation (i.e. of 360° in the same direction). The cis → trans transformation, in fact, can involve a rotation of 180° in the opposite direction to that of the trans → cis, as already discussed in relation to the catenane shown in Fig. 24.
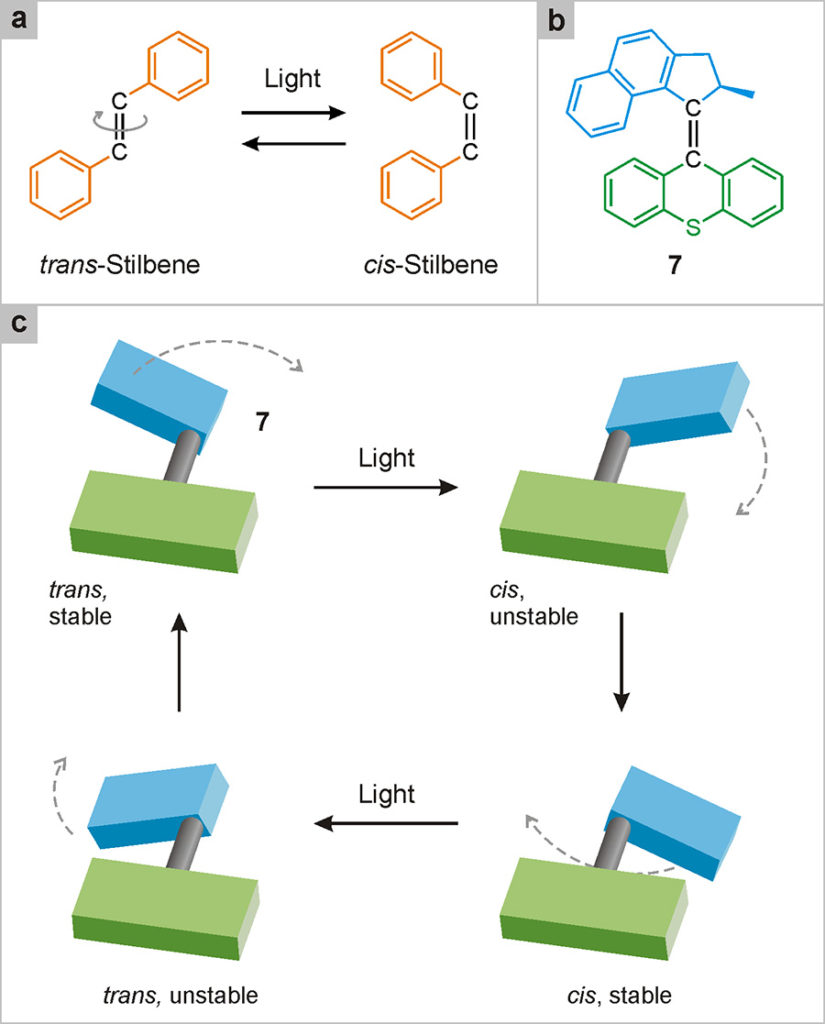
Fig. 26. Rotary nanomotors. (a) Molecules that possess the –C=C– group, like stilbene, can exist in two structurally different forms – trans and cis – interconvertible by light stimulation. (b) Molecule 7 is more complicated, but it is of the same type as stilbene; therefore, it can also exist in the trans and cis forms. The bold bond is projected above the plane of the page. (c) Thanks to an accurate design, in species 7 the absorption of two photons of light causes the rotation of 360° of one of the two units with respect to the other. Since unidirectional rotation can be repeated several times by the absorption of further photons, 7 functions as a light-driven rotary nanomotor.
However, in appropriately designed compounds containing the –C=C– double bond, one can make both the trans and the cis form not planar, but rather distorted. This peculiarity facilitates rotation in one direction with respect to the other, thus making it possible to obtain a complete rotation of one unit with respect to the other with two successive light stimuli. An example of this type is compound 7 shown in Fig. 26b, whose operating mechanism is illustrated schematically in Fig. 26c.
Following the absorption of light, the initial trans form turns into a cis structure that is unstable because it is distorted. This structure can relax (i.e., decrease its energy) spontaneously, maintaining the cis configuration, through a further rotation. The stable cis species thus obtained can absorb light, transforming itself into the distorted trans form, which subsequently evolves into the starting stable trans form, thus completing the 360° rotation.
In the last fifteen years the functioning of the molecular motor 7 and of other systems derived from it has been studied in great detail and in a wide variety of media (solutions, surfaces, liquid crystals, polymers, membranes) (Erbas-Cakmak 2015)2Erbas-Cakmak, S., Leigh, D. A., McTernan, C. T., Nussbaumer, A. L. (2015) Artificial molecular machines, Chemical Reviews, 115: 10081-10206..
As we will see in the next section, this class of rotary nanomotors has recently been used to build materials capable of contracting and expanding under the action of light.