3.2. The energy problem
We have just seen that ordered molecular movements cannot be obtained by exploiting the Brownian motion of a medium at a constant temperature. Molecular machines, like macroscopic ones, therefore need to be fed from an external source of energy.
For most machines in the macroscopic world, the necessary energy is obtained from reactions between oxygen and substances with high energy content (fuels) carried out in internal combustion engines.
We have stated that processes of this type, which among other things occur at elevated temperatures and high pressures, cannot be used to power molecular machines. They too, however, can exploit chemical reactions that take place at a constant temperature and in mild conditions. In the famous 1959 speech, Feynman noted: «[…] an internal combustion engine is impossible. Other chemical reactions, liberating energy when cold, can be used» (Feynman 1960)1Feynman, R. P. (1960) There’s plenty of room at the bottom, Engineering and Science, 23: 22-36.. This is exactly what happens in biological nanomachines, where the reactions that release the energy necessary for their functioning (typically, the hydrolysis of ATP) take place at ambient temperature and pressure and proceed through several successive stages, in each of which a small amount of energy is put at stake. Apart from these differences, the fact remains that both macroscopic and nanometric machines work by consuming a source of fuel. This inevitably leads to the formation of waste products, the elimination of which is a necessary condition for preserving the proper functioning of the machine. As we have seen (Fig. 13), nature has admirably solved this problem by recycling, through metabolism and the action of ATP synthase, ADP and phosphate in the production of new ATP (Goodsell 2009)2Goodsell, D. S. (2009) The machinery of life, New York: Springer..
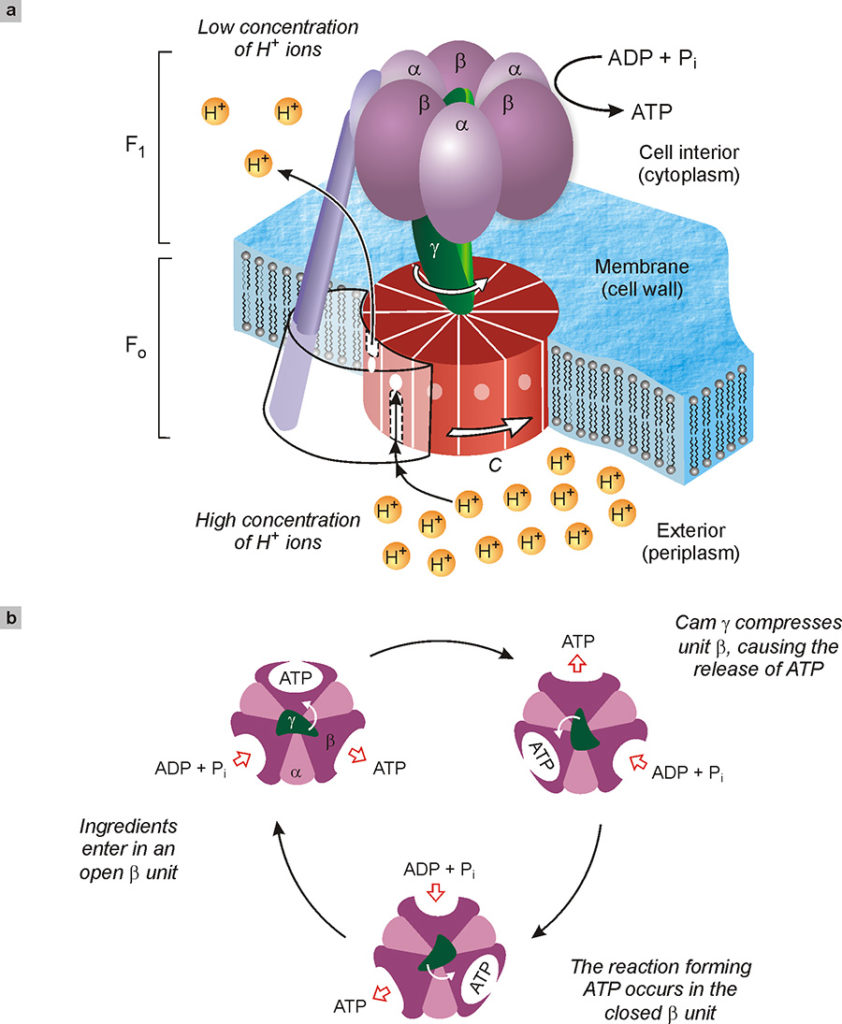
Fig. 13. Depiction of the enzyme ATP synthase tasked with the synthesis of adenosine triphosphate (ATP) starting from adenosine diphosphate, ADP, and inorganic phosphate (Pi). This enzyme, around 10 nm in size, consists of two rotating molecular motors, FO and F1, coupled together (a). In the normal functioning of the enzyme, a different concentration of hydrogen ions on both sides of the cell membrane causes a flow of the same ions through unit C. This flow rotates the unit C as if it were a mill. The γ cam, integral with C, presses successively on the catalytic units α and β of F1, causing the formation of ATP from ADP and phosphate ingredients. The top view of the enzyme (b) shows how the γ cam, by rotating, deforms in sequence the three sites where the synthesis of ATP takes place.
Research on artificial molecular machines has shown that it is possible to operate these systems not only by chemical energy, but also – and often more conveniently – by electrical or light energy (Ballardini 2001)3Ballardini, R., Balzani, V., Credi, A., Gandolfi, M. T., Venturi, M. (2001) Artificial molecular-level machines: which energy to make them work?, Accounts of Chemical Research, 34: 445-455.. These two forms of energy are particularly interesting as they allow suitably designed systems to operate without the formation of waste products. In addition, both electrical energy and light can be administered to molecules in a precisely controlled manner and with much higher resolution over time and space than chemical fuels. A further advantage of these forms of energy is that the techniques used to transfer them to molecular machines also allow us to study their functioning.
In describing artificial molecular machines we will use simplified structural formulas to indicate the chemical compounds involved and schemes to illustrate the types of mechanical movements per-formed by the machine.