1.3. Supramolecular systems
The ability of chemistry to provide “custom-made” molecules opens up new perspectives in various fields of science and technology. Each molecule has intrinsic properties that can be seen as a set of information exploitable in the interaction with other molecules or with external electrical or light stimuli. When molecules meet, each “reads” the information elements contained in the other and, de-pending on the characteristics of these elements, the molecules can either ignore each other, or react with the formation of new species, or aggregate, giving rise to supramolecular systems (Lehn 1995)1Lehn, J.-M. (1995) Supramolecular chemistry: concepts and perspectives, Weinheim: Wiley-VCH.. A supramolecular system is therefore obtained by the association of two or more molecules, which takes advantage of the so-called molecular recognition, based on specific interactions (the lock-and-key concept) such as, for example, hydrogen bonding (Fig. 6).
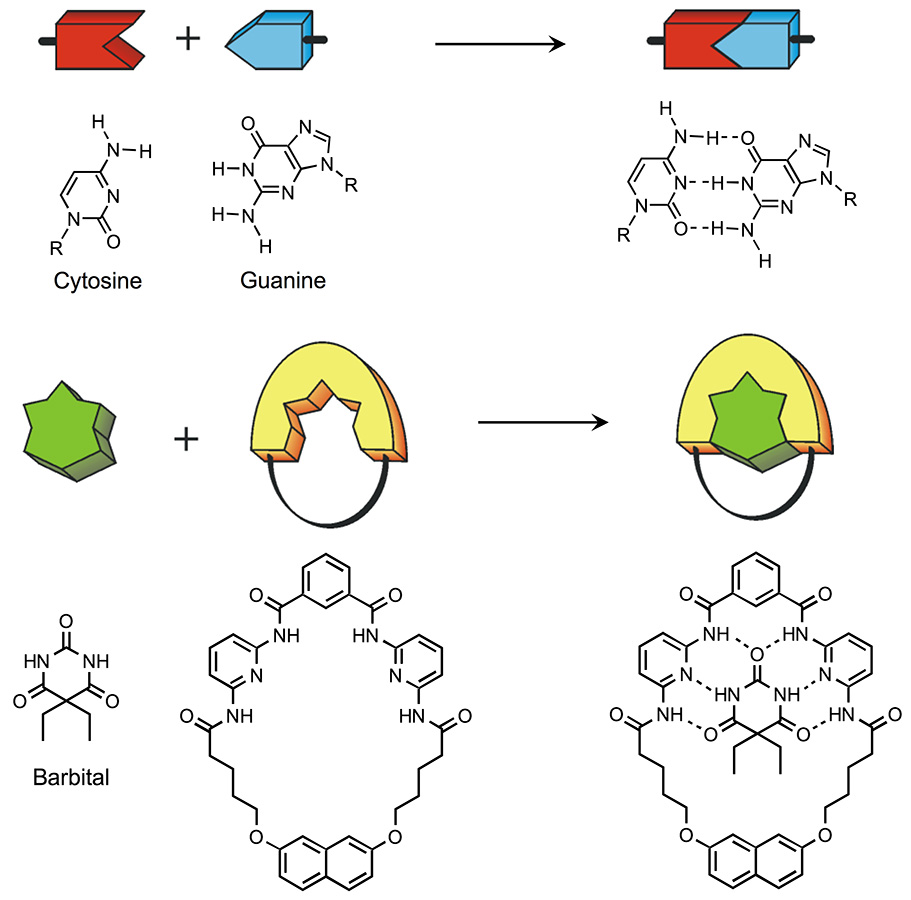
Moreover, using synthetic methods it is possible to join molecules that do not have elements of spontaneous recognition, but which can be interesting to associate for specific purposes. Therefore, supramolecular systems can be formed in various ways. If these systems are to perform interesting functions, it is necessary that the molecular components have very precise chemical and physical properties so that in the supramolecular system, thanks to the interaction between the single molecular components, new properties emerge. In this way, supramolecular chemistry becomes engineering at the molecular level and can contribute to the development of nanotechnology (Balzani 20082Balzani, V., Credi, A., Venturi, M. (2008) Molecular devices and machines: conceptsand perspectives for the nanoworld, Weinheim: Wiley-VCH.).