Continue from previous page
Invisible, but well-known
To understand chemistry, we need to have the following two concepts in mind: molecules are small, very small (nanometric), but they are three-dimensional objects that have their own specific dimension, composition, structure and shape; from these characteristics we can derive their specific properties, such as the effect on organisms.
To get a better idea of how small molecules are, just imagine that in a drop of water there are so many molecules that if we could dis-tribute them among all the inhabitants on Earth, each would receive about 200 billion. Objects of such small dimensions escape our daily experience and the common experimental investigations, so that it is difficult not only to accept their usefulness, but also to believe in their real existence. Goethe said that science must be on a human scale and opposed the use of the microscope by stating that what cannot be seen with the naked eye should not be sought, because it is obviously hidden from the human eye for some good reason. This statement is contrary to the logic of science, which particularly in recent years, has pushed its investigations increasingly towards the microscopic world, not only to learn more about nature, but also to exploit the advantages that can derive from a technological point of view.
Taken individually, a molecule can neither be seen nor weighed or measured. Despite these limitations, molecules have no hidden secrets for chemists who have, for over a hundred years, learned to distinguish, build and use them by exploiting their collective proper-ties. This concept is expressed admirably by Primo Levi whose book, The Monkey’s Wrench, gives the definition of the profession of the chemist compared to that of an engineer:
[…] My profession, my real one, the profession I studied in school and that as kept me alive so far is the profession of chemist. I don’t know if you have a clear idea of it, but it’s a bit like yours; only we rig and dismantle very tiny constructions. We’re divided into two main branches, those who rig and those who dismantle or break down, and both kinds are like blind people with sensitive fingers. I say blind because, actually, the things we handle are too small to be seen even with the most powerful microscopes: so we’ve invented var-ious intelligent gadgets to recognize them without seeing them (Levi 1978).
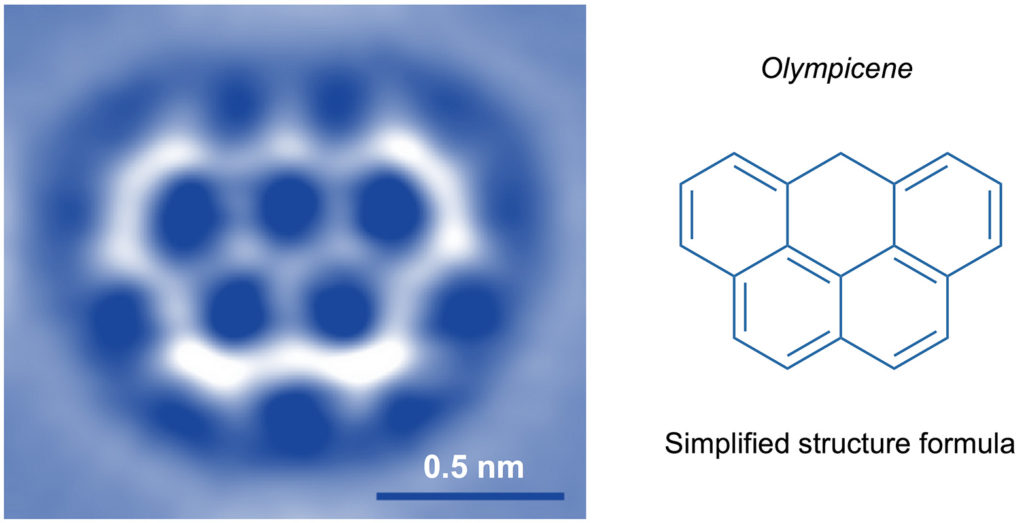
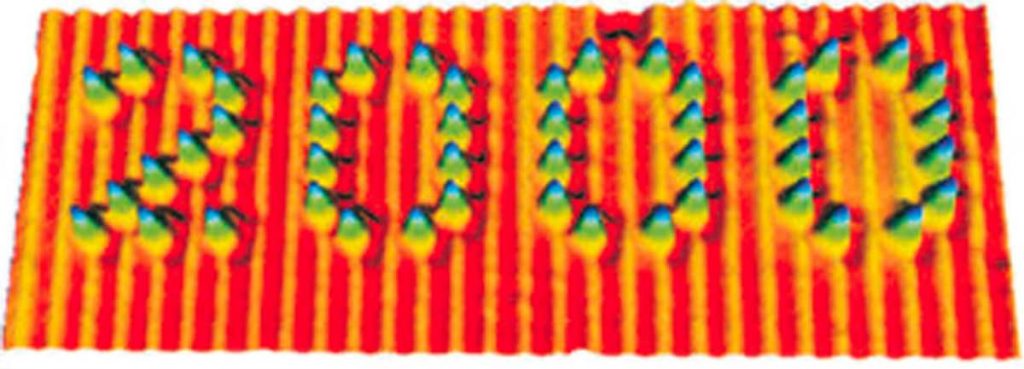
Although with the most recent advances in science it is possible to “see” (through images obtained with suitable instruments called scanning probe microscopes, Fig. 3) and even “touch” (with ultra-thin tips) single molecules to use them for “nano-writing” (Fig. 4), the world of molecules is essentially a mental representation. But it is a very objective and rational representation, as chemists have a wealth of information about molecules, such as composition, weight, size, shape, reactivity, ability to interact with light and electricity, and tendency to either remain rigidly associated with each other (solid state), slide on one another (liquid state) or move freely on their own (gaseous state).