1.1. ATOMS
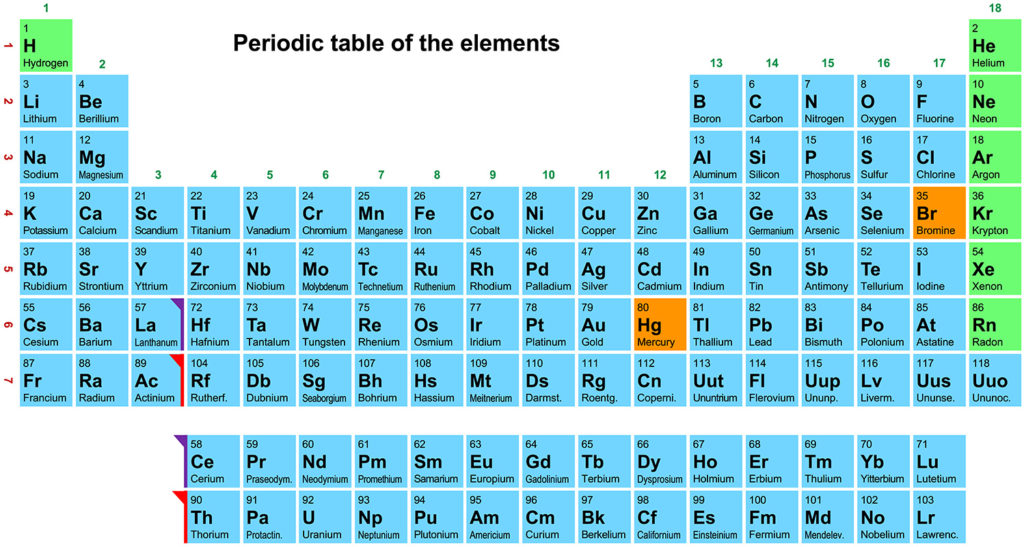
There are about one hundred elementary atomic species in nature, each represented with one or two letters of the alphabet: H for hydrogen, C for carbon, N for nitrogen, O for oxygen, P for phosphorus, S for sulfur, Cl for chlorine, Co for cobalt, and so on. These elementary species, according to the repetitiveness of their chemical and physical characteristics, are ordered in the Periodic Table or Periodic System, as coined by famous chemist and writer, Primo Levi, in his renowned book. The Periodic Table (Fig. 1) was created in 1869 by Dmitrij Mendeleev, a Russian chemist who first highlighted, even without understanding the reasons, the similarities between the properties of different elements. According to many scientists, Mendeleev’s work was the most ingenious breakthrough of the last ten centuries. For many years the Periodic Table has been regarded as something extraordinary, as if divine. Although today the reasons for the similarities between the various elements are well known, the Periodic Table still maintains its unaltered charm as the clear order of the elements provides a glimpse into the intrinsic and profound order of Nature. The Periodic Table itself contains, in a concise and unitary way, a good part of chemistry: no other scientific discipline can boast such an iconographic table.
Atoms have a spherical shape with different dimensions, depending on the type of element, and they are very small. The largest atom, cesium (Cs), has a radius of 0.24 nm (nm is the abbreviation of nanometer, which measures one billionth of a meter). In general, atoms are not isolated; they spontaneously tend to combine – that is, to form bonds – with other atoms, according to precise laws, in order to form molecules.